In this article, Sue Frankland looks at the global problem of wastage of medications, including causes such as overprescribing and non-adherence. She also presents information from her master’s dissertation on how people understand, and respond to, medication expiration dates
CLASStime: Cannabis-based medicines
CLASStime: Cannabis-based medicines

For the last decade, Martin Woodbridge has worked with regulators, and industry and health professionals to promote the rational use of cannabis-based medicines. Here, he considers the introduction of cannabis-based medicines to New Zealand and explores what it means for pharmacy practice
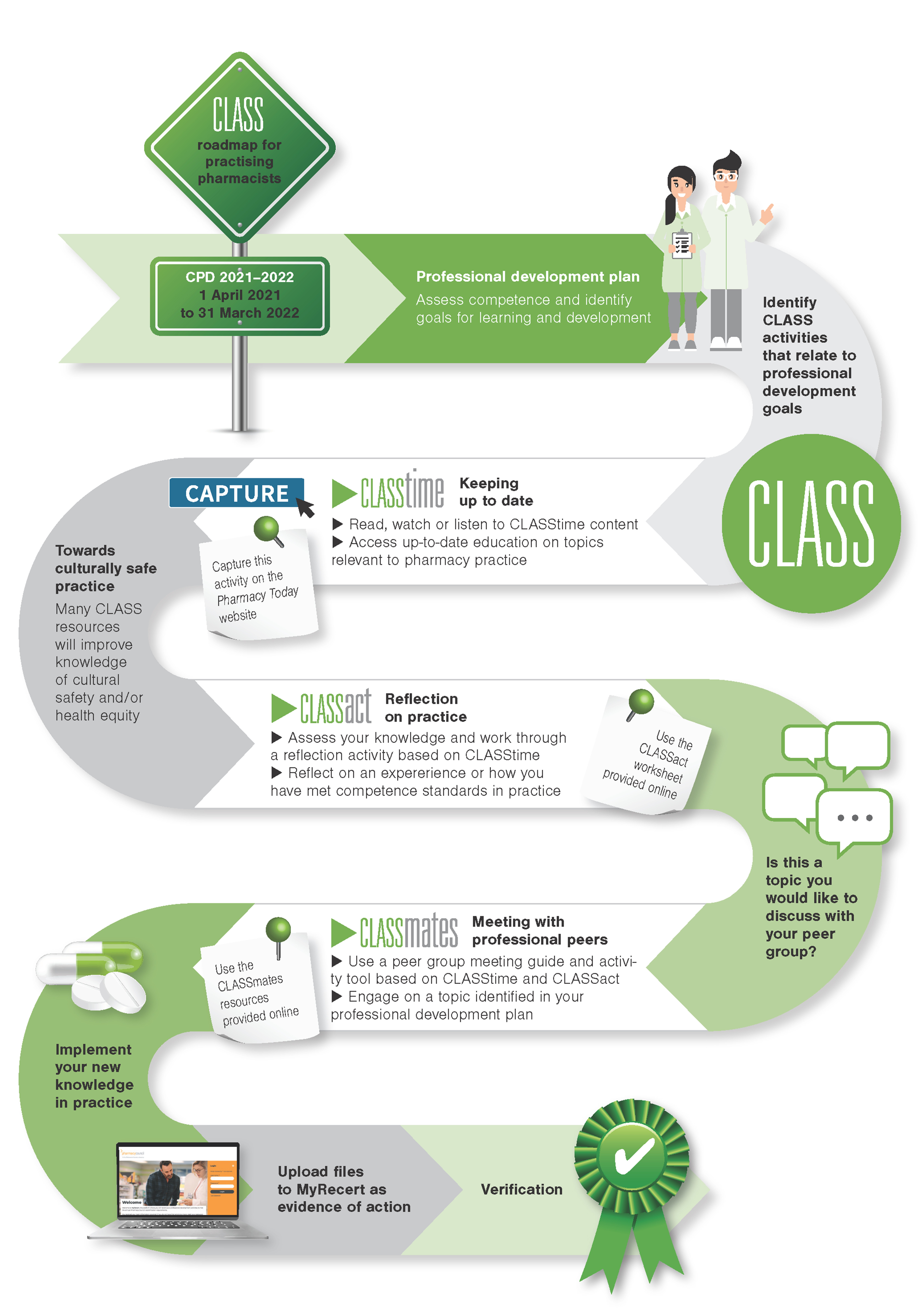
Our suite of CLASS activities provides meaningful independent and peer group learning that you can put into action, while helping you complete your annual recertification requirements.
You can choose how many elements you wish to undertake, depending on your professional development plan, but it is recommended that you complete both CLASStime and CLASSact before CLASSmates.

This CLASStime article supports professional development with respect to:
- Understanding administration and action of cannabis-based medicines
- Identifying at-risk patients, medicine interactions and adverse effects associated with cannabis-based medicines
- Providing education and advice to patients about cannabis-based medicines
As you read, highlight new knowledge or areas where you need to know more. You can also use the Capture button online to keep notes of your thoughts and reflections on this article. This may be useful if you choose to proceed to CLASSact and CLASSmates for this topic.
Completing this CLASStime activity may allow you to fulfil some or all of the following elements of your Pharmacy Council annual recertification requirements:
- Keeping up to date – go to MyRecert (myrecert.pharmacycouncil.org.nz), select the “Keeping up to date” page of your portfolio, then enter a description of this article
- Reflection on practice
This activity has been endorsed by the Pharmaceutical Society of New Zealand as suitable for inclusion in a pharmacist's continuing education records for continuing professional development purposes.
A variety of standardised cannabis-based medicines and modes of administration are available globally. It is likely New Zealand will have access to these, and similar medicines produced domestically, as more products are assessed by the Medicinal Cannabis Agency and Medsafe. Pharmacists and prescribers need to work collaboratively as cannabis-based medicines continue to be introduced into New Zealand.
Pharmacists need to start planning for the future now, including thinking about the following questions:
- What role will you play in patient care, given these are new, often untested medicines?
- How will cannabis-based medicines impact on your service, and how will you adapt to meet that need?
- What innovations will be required in your pharmacy practice to promote wellbeing of patients using cannabis-based medicines?
Cannabis comprises a single species of dioecious plant, Cannabis sativa L., one of the oldest known medicinal plants. Cannabis-based medicines are derived from cannabis, with defined and standardised cannabinoid content (eg, tetrahydrocannabinol [THC] and cannabidiol [CBD], which act on the cannabinoid receptors) in standard dosages.
As of 1 October, cannabis-based medicines distributed in New Zealand must meet the Medicinal Cannabis Agency minimum quality standards or be approved or provisionally approved by Medsafe. The minimum quality standard aims to standardise medicines to assure dosage composition, repeatability of dose, and the ability to adjust dose by titration. To ensure patient safety, these products should be free of contaminants such as microbes, pesticides, impurities and heavy metals.1
A list of verified and approved products is available on the Ministry of Health website (tinyurl.com/MedCannabisProducts). All medical practitioners are able to prescribe these products. Unverified products can be prescribed/imported for a named patient with ministry or chief medical officer approval.1
In New Zealand, all CBD products are prescription medicines. A CBD product may contain only trace amounts of THC (the cannabinoid with psychoactive effects). Any product made from a cannabis extract which is not a CBD product (ie, THC products) is a Class B1 controlled drug, on prescription. The cannabis flower (fruit) and any part of any plant (eg, leaves) containing cannabis resin (ie, glandular trichomes containing THC) are Class C1 controlled drugs, on prescription.
Only a few cannabis-based medicines are officially approved (eg, in New Zealand, Sativex oromucosal spray) for specific conditions.
The prescription of unapproved medicines, or prescribing off label, requires a good clinical argument. It then becomes the dispensing pharmacist’s role to ensure the medicines prescribed are suitable, to advise patients about the medicine (how to take them, what reactions may occur) and to answer patient questions (which could be few or many).
Like opioid receptors reacting to opioid agonists (morphine, codeine, oxycodone), humans have a distinct receptor system for cannabinoids. Phytocannabinoids produced by C. sativa L. (THC and CBD) elicit their effects by binding to specific cannabinoid receptors, which belong to the superfamily of G-protein-coupled receptors. Two types of cannabinoid receptors – CB1 and CB2 – have been identified with certainty.
Phytocannabinoids work in a similar way to our naturally produced endocannabinoids, which are neurotransmitters with biological activities within the human endocannabinoid system. The ECS is involved in a host of homeostatic and physiologic functions. It plays a critical role in our nervous system and regulates multiple physiological processes, including the adjustment of our response to pain, appetite, digestion, sleep, mood and memory.
Pharmac’s Pharmacology and Therapeutics Advisory Committee (PTAC) recently considered “medicinal cannabis”, which they defined as a prescription-only plant component, extract or processed product derived from C. sativa, for therapeutic use, with a defined content of CBD and THC. In the report, PTAC raise two very important points:2
- The full cost of medicinal cannabis is currently privately funded (out-of-pocket payments).
- There may be an unmet need for effective, accessible therapy and, therefore, there may be an interest in the use of medicinal cannabis in a range of often complex conditions.
These conditions include, but are not necessarily limited to: degenerative conditions; epilepsy, including paediatric epilepsy; chronic mental health conditions, such as PTSD or severe anxiety; chronic pain, including neuropathic pain; spasticity associated with multiple sclerosis; and autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease and chronic fatigue syndrome.2
The two points made by PTAC have implications for prescribing and dispensing cannabis-based medicines. First, patients self-fund the medication for the duration of that prescribed treatment. Second, patients are likely to present with complex health conditions, on multiple medications, at various stages of disease progression. For pharmacists, this raises issues around:
- dealing with potential medicine–medicine interactions
- managing cannabis-based medicine initiation and titration to an optimal dosage
- potentially, cessation of the medicine.
Patients may want to stop taking a cannabis-based medicine due to the ongoing costs or if the medicine is found not to be efficacious or to cause intolerable side effects. Remember that the rational use of medicines requires that “patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community”.
The form in which a cannabis-based medicine is administered determines the onset, intensity and duration of effects (how it moves and works in our body – see table).
Like other medicines, cannabis-based medicines are available in different dose forms. This is because the dose form can influence patient behaviour in different ways, including:
- whether people actually take their medicine and adhere to their daily regimen
- when the medicine is taken (time of day)
- how often it is taken (frequency of use)
- how much is taken (total daily dosage)
- side effects and how these are tolerated
There are two major administration routes – inhalation and oral.
Inhalation
The immediate onset of action means inhalation is the preferred choice for many patients.
Given the risks from smoking, patients now seek reliable, affordable and portable vaporisers or inhalation devices. Research dedicated to advancing vaporiser and inhalation technology has seen major developments in device quality.
Using a vaporiser medical device, cannabinoids are inhaled (from dried cannabis inflorescence/flos/flower) as a vapour, and then enter the bloodstream from the lungs.
These medicines are not yet available in New Zealand (tinyurl.com/MedCannabisProducts).
Oral and oromucosal
Oral preparations are familiar dose forms (eg, tablet, capsule, oral liquid). They are similar to other medicines that patients already take, and they are easy to administer. As a result, concentrated cannabis extracts are becoming increasingly popular.
Cannabinoids (whole plant extracts or individual cannabinoids) are taken by mouth and either swallowed (oral) or applied to the oromucosal surface (sublingual/buccal). When swallowed, the medicine enters the bloodstream via the stomach, intestines and liver. When absorbed from the oral mucosa, the medicine bypasses the liver and enters into the bloodstream directly. Consequently, absorption and onset of action are faster via the sublingual/buccal route.
Pharmacists can assist patients with managing their medicine-use behaviours and ensuring their safety. When introducing cannabinoids into a patient’s therapeutic regimen, pharmacists should consider the following.
At-risk patients
Consider potential risks in older people (eg, postural hypotension), young people (given the potential risk to the developing brain), and in those with cardiac risk factors (cardiopulmonary disease) or a history of psychosis or depression.
The use of cannabis-based medicines is not advised in pregnancy or during breast feeding. A reliable contraception is advised for the duration of, and several months after, treatment.
Absolute contraindications include acute psychosis and other unstable psychiatric conditions. Relative contraindications include severe cardiovascular disease (may exacerbate arrhythmia or a history of arrhythmias), immunological, liver or kidney disease, especially in acute illness.
Potential medicine interactions
Medicines can interact with each other. The risk increases if a patient is taking many medicines at once. Patients prescribed cannabis-based medicines may have complex conditions and take multiple medicines.
A number of medicines potentially interact with cannabis-based medicines. Pharmacists should be aware of potential medicine–medicine interactions and how these will be identified and treated.
Care should be taken when cannabis-based medicines are co-prescribed with medicines that:
- interact with the cytochrome P450 (CYP450) metabolic enzymes, as these also metabolise cannabinoids (THC and CBD are metabolised by CYP3A4 and CYP2C9; CYP3A4 inhibitors slightly increase THC levels; CYP3A4 inducers slightly decrease THC and CBD levels; CBD, but not THC, is metabolised by CYP2C19)
- have sedating effects (also co-administration with alcohol), as the combination can affect response time, coordination and concentration
- influence heart and circulation (eg, beta-blockers, diuretics, adrenaline), as THC influences blood pressure
- have action at the opioid receptors, as THC appears to enhance the action of opioids (eg, codeine, morphine).
This list is not exhaustive. A full review should be undertaken before co-prescribing, in particular, those medicines that interact with the CYP450 metabolic enzymes.
Potential side effects
In general, patients seem to tolerate cannabis-based medicines well. However, some patients taking cannabinoids have worsened symptom control and new side effects, such as sleepiness and diarrhoea, or have affected normal liver function. In particular, large doses of THC have been shown to pose a risk of harm (eg, postural hypotension resulting in a fall).
There are also many unknowns with large doses of CBD – indeed, the US Food and Drug Administration continue to monitor their use, given “there are many unanswered questions about the science, safety, and quality of products containing CBD”.3
Side effects mainly occur after taking high doses or when used in combination with other substances. These tend to occur quickly after use. For THC-dominant medicines, these include:
- dry mouth
- redness of the eyes
- heightened appetite (which may be desirable)
- mild euphoria
- reduction of alertness of the user, especially in the few hours directly after consumption
- increased heart rate
- lowering of blood pressure and dizziness.
In general, all side effects will slowly decrease and then disappear within a few hours. This depends on the dose taken and mode of administration.
Titration of dose
Like with other medicines, individual patients will respond differently to cannabis-based medicines. Their response depends on the cannabis product used, the condition being treated, the duration of treatment, how it is administered and genetic predispositions.
A patient’s doctor generally provides advice regarding dose titration (dose adjustments to a desired effect) to achieve an optimal daily dosage. Titration helps patients to obtain the desired therapeutic effects and to minimise undesired effects.
Many patients will titrate their dose in the first weeks of therapy. Pharmacists should provide guidance, as per the prescription, on:
- the starting dose and titration up and down (minimum and maximum dose), including how dosage adjustments would be made based upon the dose form
- how to find an optimal daily dosage based on the severity of the patient’s condition and changes in their other medication
- how to maintain their daily dosage.
Keep in mind that the pharmacological effects are dose related and subject to inter-patient variability. Systemic absorption will be different in intensity and timing, depending on the route of administration (refer back to table).
Medicine safety
Be cognisant of possible medicine and food interactions, and potential for adverse reactions, based on the patient’s total medicine regimen. Discuss ways to reduce the risk of side effects occurring. Explain that these are novel medicines and there may be yet unknown adverse reactions to these medicines, which should be reported.
Encourage your patient to tell you about any adverse effects. Report these immediately to the Centre for Adverse Reactions Monitoring within the New Zealand Pharmacovigilance Centre (nzphvc.otago.ac.nz/reporting). The more details provided, the better – the medicine name and dose suspected of causing the reaction is necessary, alongside a list of symptoms, signs, laboratory results and past medical history.
Patients should avoid driving, operating machinery or engaging in any potentially hazardous activity under the influence of cannabis-based medicines. THC and CBD may produce undesirable effects, such as drowsiness, which will impair judgement and driving performance.
THC-containing medicines are often considered desirable psychoactive substances. Like other controlled drugs, these medicines require the same guidance and considerations by pharmacists to limit diversion and misuse.
Cannabis-based medicines should be stored in the official pharmacy container, in a safe and secure place where young children cannot reach, and away from heat and direct sunlight (eg, in accordance with label storage conditions).
If travelling internationally, patients should be reminded to check that it is legal for them to take cannabis-based medicines into any countries they are travelling to or through. Legal status will vary between countries.
Medicine efficacy
Request feedback from your patient regarding side effects, and determine if the desired effect has been obtained and maintained over time. In direct consultation with the prescribing doctor, discuss a plan to modify or stop treatment at a particular dosage if no significant effect has been seen, if intolerable side effects have occurred, or if dependence has been identified.
Returns protocol
Confirm a protocol for returning unwanted or unused cannabis-based medicine and, if required and necessary, the device used to administer it. Out-of-date or unwanted medicine should be taken back to the pharmacy for safe disposal.
The different medicine use settings (hospital, hospice and home) each present pharmacists with unique challenges. For a public hospital or hospice pharmacy, in particular, there are a number of logistical hurdles with the introduction of cannabis-based medicines. These might include the following.
Cannabis-based medicine is privately funded but may be dispensed by a publicly funded hospital pharmacy, meaning the cost of dispensing falls on the pharmacy without remittance.
Standard medicine dispensing methods and audits are difficult to employ (including how these medicines are disposed of or destroyed) because cannabis-based medicines are not always presented in standard-dose formats. As a result, it is difficult to evaluate dispensing efficiency compared with/alongside other dispensed medicines.
Recording medicines usage (as specific doses) is difficult because medicines may not be presented in standardised formats (eg, oils in resealable dropper bottles or taken by syringe from resealable bottles; inhaled doses as a granulated cannabis flower, with non-specific unpackaged dose weights) or are brought into a hospital setting and presented to pharmacy open and partially used. This makes it difficult to measure/quantify the amount of medicine available and used.
Inhaled dose forms (pulmonary administration via a medical vaporiser device) are also a hurdle because of the following factors:
- There is currently no standardised dose (ie, a pad for insertion into a vaporiser), which makes it difficult to audit administration and usages.
- A general policy of 100 per cent occupancy for public or private hospitals or hospices would mean administration by vaporisation would occur in rooms with a minimum dual occupancy (outside of pandemic conditions); second-hand exposure to exhaled vapours may present an issue for nursing staff (especially if pregnant), so should be administered in a well-ventilated space/room.
Further considerations for all pharmacists, including community pharmacists:
- Oral dose forms may require cold chain supply and, thus, storage in a locked pharmacy refrigerator. This is both a cost and storage compliance burden.
- Inhaled dose forms require a medical device for administration (eg, a vaporiser) to be registered as a medical device in New Zealand or by an approved overseas medicine regulator (eg, Syqe Medical and Storz & Bikel). Furthermore, there is poor knowledge of vaporisation technology and its safe use among pharmacy and nursing staff, and among many patients.
Forget the hype – cannabis-based medicines are not a panacea or a cure for disease. Focus on providing good advice to patients – they need a supportive but critical view of the risks and benefits of cannabis-based medicines.
For pharmacists, dispensing cannabis-based medicines is similar to other medicines. However, many patients will have an opinion about cannabis. Some patients will be armed with information, whereas others will be naive to the topic.
A solid understanding of these medicines and clear communication are really important. In particular, pharmacists must be able to:
- answer tricky medicine queries
- promote patient medicine compliance and share patient-friendly medicine information
- assist in identifying side effects and adverse reactions – report them and encourage patients and carers to inform their doctors
- support prescribers in decision-making and with medicine regimen review.
When talking with patients, first, ask the patient what they already know about cannabis-based medicines. Inform them about the mechanism of action, how to use it, the dosage regimen, possible side effects and how to safely store it.
Make sure the patient takes notice of contraindications – certain conditions where cannabis-based medicines should not be used – and possible interactions with other medicines.
In a follow-up discussion, ask the patient about their experience with the medicine, with extra attention to side effects and the medicine’s effectiveness.
NOW AVAILABLE ONLINE: Fleisch J, Woodbridge M. A clinical primer: A guide to the rational use of cannabis-based medicines. 2022.
Martin Woodbridge is a research, technical advisory and policy consultant who has undertaken projects with primary healthcare, ministries of health, universities, the pharmaceutical industry and front-line health services here and abroad
Please CAPTURE this article before leaving this page. To move on to CLASSact, where you can download an interactive PDF that provides you with both knowledge and reflective questions for independent learning, click the button below.
Completing this CLASSact worksheet may allow you to fulfil some or all of the following elements of your annual recertification requirements:
- Keeping up to date
- Reflection on practice

Please CAPTURE this article before leaving this page. To move on to CLASSmates, where you can access a peer group meeting guide and activity tool based on CLASStime and CLASSact, click the button below. It includes a facilitator guide, user guide, notes template and Jamboard tool (for online meetings).
Completing this CLASSmates activity may allow you to fulfil some or all of the following elements of your annual recertification requirements:
- Keeping up to date
- Reflection on practice
- Meeting with professional peers
1. Ministry of Health. Medicinal Cannabis Agency - Minimum quality standard. 1 October 2021. tinyurl.com/MOHMedCannMQS
2. Pharmacology and Therapeutics Advisory Committee. Record of the Pharmacology and Therapeutics Advisory Committee Meeting. 25 March 2021. pharmac.govt.nz/assets/ptacminutes-2021-03.pdf
3. US Food and Drug Administration. What You Need to Know (And What We’re Working to Find Out) About Products Containing Cannabis or Cannabis-derived Compounds, Including CBD. tinyurl.com/FDAMedCannabis
NOW AVAILABLE ONLINE: Fleisch J, Woodbridge M. A clinical primer: A guide to the rational use of cannabis-based medicines. 2022. https://bedrocan.com/about-us/download/